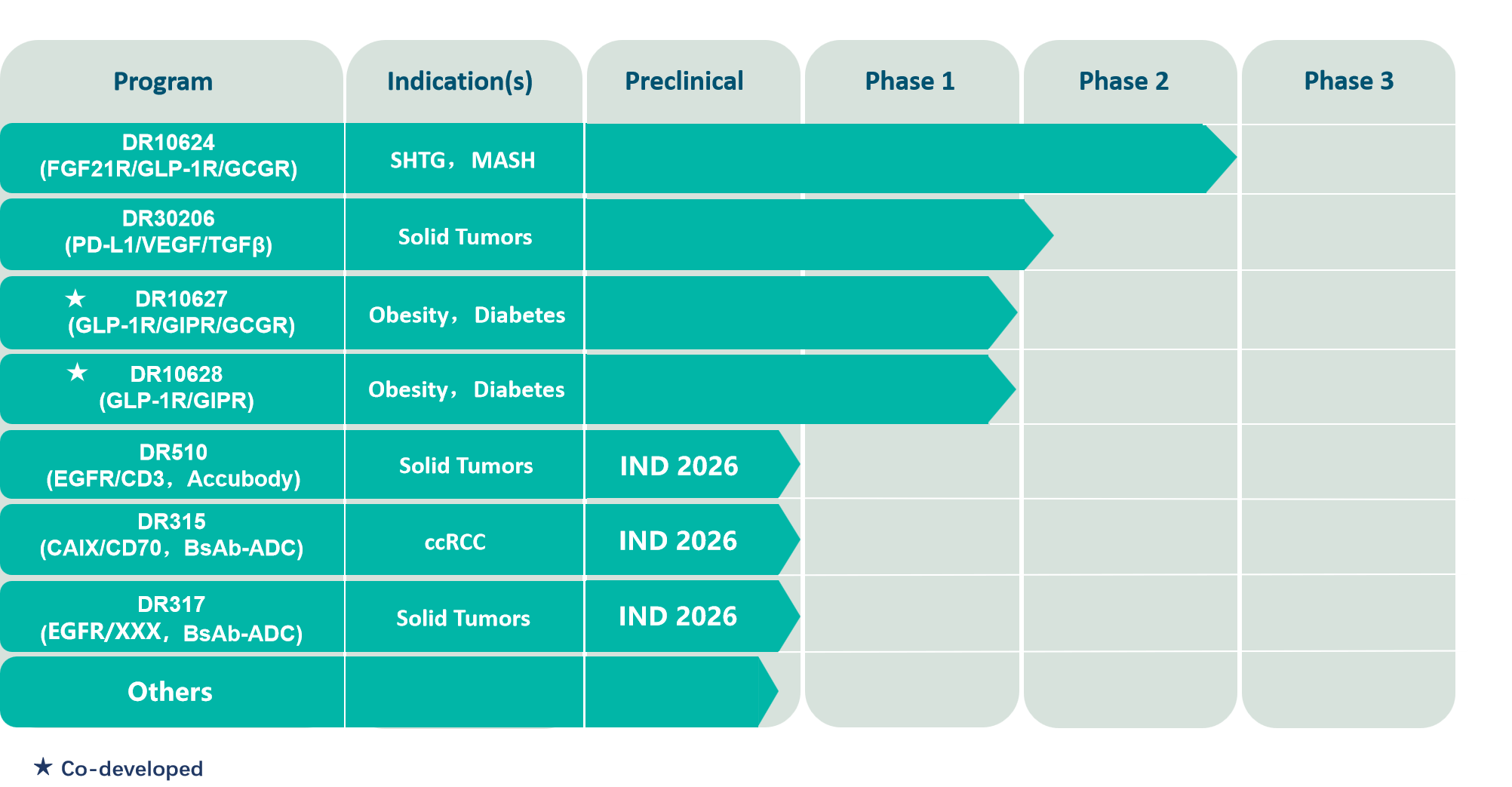
DR10624 is designed using the MultipleBody® technology platform. It is a trispecific fusion protein created by combining a highly stable FGF21 analog, a GCGR/GLP-1R dual-agonist peptide, and a human IgG Fc. DR10624 received approval in April 2022 from the New Zealand Medicines and Medical Devices Safety Authority (Medsafe) and the Health and Disability Ethics Committee (HDEC) to initiate Phase I clinical trials locally. Clinical trials of DR10624 in China were approved in July 2023, and Phase II clinical studies have now been completed. The results were presented in an opening session report at the 2025 AHA. Clinical results show that DR10624 can significantly and rapidly reduce triglycerides (TG). In patients with severe hypertriglyceridemia (SHTG), a rapid decrease in TG was observed after the first dose of DR10624, and low levels were maintained throughout the 12-week treatment period. Furthermore, DR10624 demonstrated excellent efficacy in improving atherogenic lipid profiles, a significant increase in adiponectin levels (suggesting substantial improvement in insulin sensitivity), a significant reduction in uric acid levels (beneficial for organs such as kidney and heart), and weight loss effects. DR10624 has a favorable safety profile. These positive clinical data support future larger-scale Phase III clinical trials for DR10624 to further explore its therapeutic potential in patient populations with SHTG, mixed hyperlipidemia, and metabolic dysfunction-associated steatohepatitis (MASH).
DR30206 is a trispecific fusion protein targeting PD-L1, VEGF, and TGF-β. It aims to treat tumors by blocking the PD-1/PD-L1 signaling pathway, neutralizing free VEGF and TGF-β, thereby restoring the proliferation of exhausted T cells, reducing tumor angiogenesis, and alleviating immunosuppression in the tumor microenvironment (TME). The TME of solid tumors is exceptionally complex, with multiple concurrent immunosuppressive pathways. The trispecific design of DR30206 is expected to improve the immune environment within the TME and enhance anti-tumor efficacy. Clinical trials of DR30206 in China were approved in June 2023, and multiple Phase Ib and IIa clinical studies are currently ongoing.
DR10627 is created by conjugating a GLP-1R/GIPR/GCGR triple-agonist peptide to a fatty acid chain. The peptide sequence was extensively screened to optimize agonism of the three targets to the greatest extent. In preclinical animal studies, DR10627 demonstrated remarkably significant efficacy in lowering blood glucose and reducing body weight. Its indications are obesity and diabetes. Clinical trials of DR10627 in China were approved in January 2022, and Phase I clinical studies have now been completed.
DR10628 is obtained by cross-linking a peptide with dual agonistic activity at GLP-1R and GIPR to a long-chain fatty acid chain. In preclinical animal studies, DR10628 demonstrated remarkably significant efficacy in lowering blood glucose and reducing body weight. Its indications include metabolic diseases such as obesity, diabetes, and MASH. Clinical trials of DR10628 in China were approved in April 2023, and Phase I clinical studies have now been completed.
DR510 is a prodrug-type T-cell engager (TCE) targeting human EGFR and CD3, developed based on Doer Biologics' proprietary Accubody® technology platform. DR510 utilizes a self-developed cleavable substrate linker and a VHH that specifically neutralizes the aCD3 scFv as an activity-shielding domain (VHH masking domain, VHHm). DR510 is activated by cleavage at a single site, releasing the TCE and the ABD-VHHm, thereby enhancing therapeutic activity while ensuring minimal off-target toxicity in the periphery. The Accubody® platform distinguishes itself from most previously reported TCE prodrugs by not requiring the screening of different masking peptides for dual masking of the tumor antigen and CD3-targeting antibodies. Preclinical in vitro and in vivo results indicate that DR510's unique dual-masking design achieves a critical balance between efficacy and safety. DR510 plans to submit an IND application in 2026 for the treatment of EGFR-positive solid tumors.
DR315 is a bispecific antibody-drug conjugate (ADC) targeting CA9 and CD70. It leverages the differential expression of CA9 and CD70 in normal tissues to reduce on-target toxicity, particularly the potential hematological toxicity associated with clinically reported CD70-targeting ADCs, thereby improving the therapeutic window. Its indication is renal clear cell carcinoma. DR315 employs a novel linker-topoisomerase inhibitor payload. In preclinical in vitro and in vivo studies, DR315 demonstrated excellent tumor cell killing and inhibitory activity. NHP pre-toxicology studies indicate a favorable safety profile. DR315 plans to submit an IND application in 2026.
DR317 is a bispecific ADC drug targeting EGFR/XXX, intended for the treatment of various solid tumors, especially non-small cell lung cancer (NSCLC) resistant to third-generation TKIs and platinum-resistant ovarian cancer. DR317 enhances selectivity for EGFR/XXX double-positive tumor cells by modulating antibody affinity and utilizes a novel linker-topoisomerase inhibitor payload. Through its efficient bystander effect, it further addresses tumor heterogeneity. In preclinical animal trials, DR317 demonstrated outstanding anti-tumor effects. The NHP pre-toxicology experiments indicated a favorable safety profile. It plans to submit an IND application in 2026.